Hard Water 101 @welshiecurlgirl
Hard Water 101 @welshiecurlgirl







I recommend using a chelating shampoo instead of the listed alternatives, it is easy to mess up hair if you don't have the proper pH. A chelating shampoo will be the proper pH and is effective.
Taken from:
https://www.instagram.com/p/CkZHgkDIM-6
The showerstik is the only shower head that can actually filter out the minerals that I know of, regular shower heads can help remove chlorine but will not make water soft.
Hardness prevents soap from lathering by causing the development of an insoluble curdy precipitate in the water; hardness typically causes the buildup of hardness scale (such as seen in cooking pans). Dissolved calcium and magnesium salts are primarily responsible for most scaling in pipes and water heaters and cause numerous problems in laundry, kitchen, and bath.
Symptoms of Hard Water include:
- Stiff, dingy laundry
- Mineral deposits on dishes and glassware
- High soap usage & need for fabric softeners
- Extra work to remove soap curd on bathtubs & shower stalls
- High energy costs, possibly due to scale build-up in pipes and on appliances
- Scale build up in sinks, tubs, faucets & appliances
Taken from:
https://wqa.org/Learn-About-Water/Perceptible-Issues/Scale-Deposits/
Map of Hard Water in the USA (USGS):
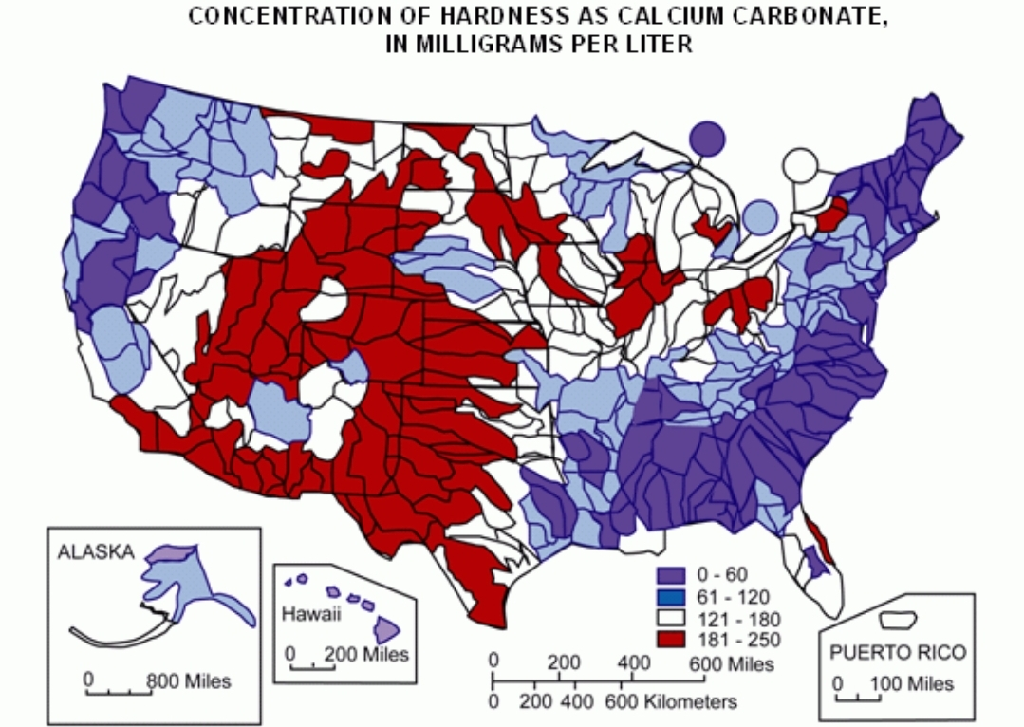
General guidelines for classification of waters are:
- 0 to 60 mg/L (milligrams per liter) as calcium carbonate is classified as soft
- 61 to 120 mg/L as moderately hard
- 121 to 180 mg/L as hard
- More than 180 mg/L as very hard.
Taken from:
https://www.usgs.gov/special-topics/water-science-school/science/hardness-water#overview